Waldenström Macroglobulinaemia (WM) is an indolent lymphoma still primarily managed with chemo-immunotherapy, which remains a key first-line treatment option, although recently BTKIs have emerged as potential therapies for untreated patients.
The aim of our study was to evaluate and compare efficacy and safety of different chemo-immunotherapeutic regimens commonly used in Italy for frontline WM treatment.
A retrospective analysis was conducted on 547WM patients enrolled between 2008-2022 from 14 Italian haematological centres. Among them, 245 received the BR scheme (bendamustine-rituximab), 116 were treated with DRC (dexamethasone-rituximab-cyclophosphamide), and 129 were treated with various other regimens including monotherapy with chlorambucil or cyclophosphamide as well as more aggressive combinations, such as Chl-R (chlorambucil-rituximab), FCR (fludarabine-cyclophosphamide-rituximab) or R-CHOP (rituximab-cyclophosphamide-doxorubicin-vincristine-prednisone), 48 patients received rituximab monotherapy for neurological symptoms.
The main focus of our analysis was on the two major treatment groups: BR and DRC.
There were notable differences between these two groups in terms of age at treatment (69 vs 70 years old, respectively), CIRS (more patients with CIRS>6 in DRC) and revised IPSSWD (higher risk rates in BR). Patients treated with BR tended to be younger, more fit but also had a more aggressive disease.
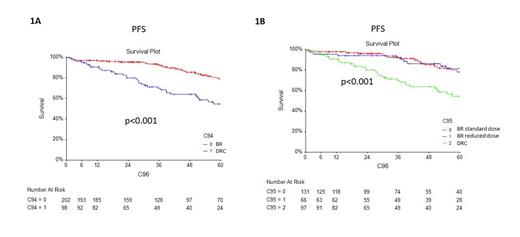
Overall survival (OS) curves did not show significant differences between the two schemes (5-year OS 87.5% for BR and 93.0% for DRC, p=0.42). Similarly, the analysis of progression free survival (PFS) curves demonstrated a better PFS at 5 years for BR (79.2%) compared to DRC (54.5%) (p<0.001; figure 1A). The median PFS was 63.6 months for DRC, while it was not reached for BR. When analysing BR-treated patients separately (standard dose and reduced initial dose) both groups showed better PFS curves than DRC (p<0.001; figure 1B).
Multivariate analysis identified the choice of the treatment (BR vs DRC) as the sole significant variable impacting PFS (RR 1.76).
Notably, approximately 33% of the patients treated with BR received an initial bendamustine dose lower than the standard dose of 90 mg/m 2 for first-line therapy with 91% of these cases receiving 70 mg/m 2. The two schemes BR and DRC were well tolerated with no significant reduction in administered cycles (16.3% for BR vs 20.7% for DRC). However, dose reduction was more common for BR (14.3%) compared to DRC (6.0%).
In conclusion, this large Italian retrospective real-life study showed excellent outcomes for unselected WM patients treated with chemo-immunotherapy. Despite the emergence of new treatment options, chemo-immunotherapy remains a highly effective treatment modality for these patients. The long-term follow-up confirmed better PFS for BR-treated patients, while OS, remained comparable between BR and DRC. Moreover, the BR scheme exhibited excellent PFS even with a reduced initial bendamustine dose.
Disclosures
Tedeschi:Astrazeneca: Speakers Bureau; Abbvie: Speakers Bureau; Beigene: Speakers Bureau; Janssen: Speakers Bureau. Ferrero:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy; Sandoz: Consultancy; Beigene: Research Funding; Morphosys: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; Clinigen: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gentili: Speakers Bureau; Italfarmaco: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal